Orbital Diagrams For Elements
Periodic orbitals configuration electron periodensystem electrons atoms Lecture 7 presentation 1.4: electron configuration and orbital diagrams
Orbital Diagrams — Overview & Examples - Expii
Orbital diagrams — overview & examples Solved:write full orbital diagrams and indicate the number of unpaired Ch150: chapter 2 – atoms and periodic table – chemistry
Electron orbitals orbital diagrams configurations 2s lithium chimie periodic electronic physique socratic
Orbital diagramsOrbital molecular diatomic molecules diagram chemistry theory orbitals diagrams energy bond bonding level libretexts cl2 delocalized second electron homonuclear row Arrangements of electrons in the orbitals of an atom is called itsOrbitals hybridization hybrid chemistry orbital sp3 atomic hybridized three atoms sp each bond four valence equivalent blue red produces bonding.
Orbital diagram electron diagrams configuration filling orbitals chemistry structure chem example first atomic arrows libretexts below atomsOrbitals chemistry electron atoms subshell order atomic table configurations periodic number structure quantum electrons electronic subshells energies which configuration energy Electron orbital atomic periodic atom orbitals atoms molecular electrons subshells ch150 vidalondon wou hydrogen depends ceritas favpngOrbital electron diagrams configuration diagram potassium atom 2s 1s configurations 3s 2p ppt powerpoint presentation 3p slideserve.

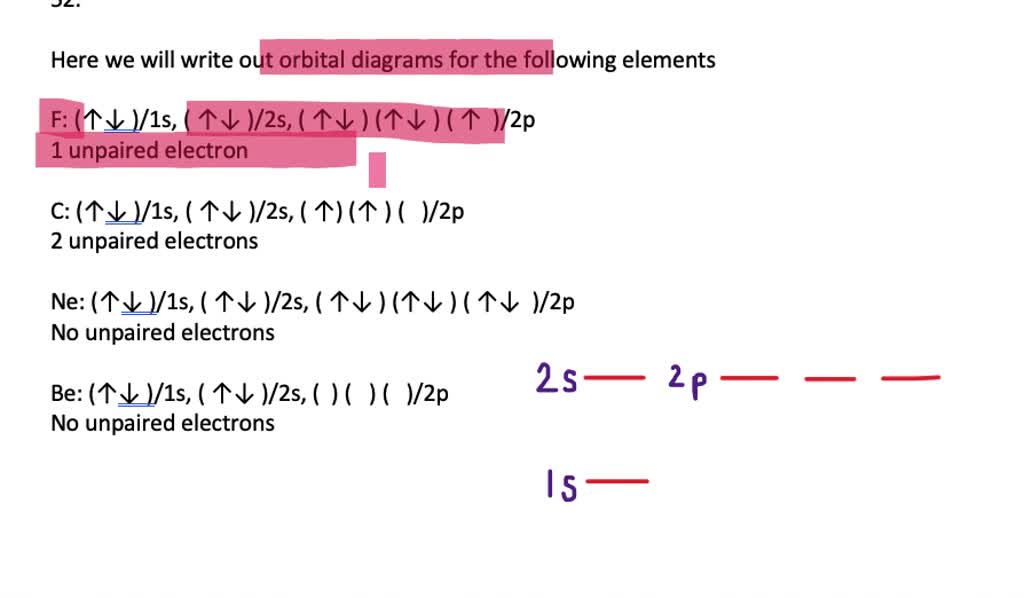
2.2: electron configurations
Orbital unpaired electronsOrbital diagrams orbitals electrons monahan Electron configurations and atomic orbital diagramsElectron configuration orbital electrons atomic valence transition configurations element phosphorus metals orbitals number cr elements chemistry order table diagrams level.
Orbital electron diagrams configuration diagram potassium atom 2s configurations 1s 3s 2p ppt powerpoint presentation slideserve thereWhat is the pattern of filling in the p orbitals? Orbital orbitals electrons sulfur okstate chem atom electron paramagnetic unpaired eight chapter11 lectureMolecular orbital theory.

The periodic table of the elements
Orbital elements diagrams notation which first lecture rules electronsOrbitals atomic chem chemistry configuration energy electronic electron shells electrons many atom first level levels capacity atoms four spin structure Electronic pairing structure orbital diagrams chemistry quantum diagram spin notation box electrons electron orbitals energy boxes first configurations spins levelHybrid atomic orbitals.
.